Abstract
Up to 70% of large B-cell lymphoma (LBCL) patients will eventually experience relapse or progress following CD19-CAR-T therapy. Data guiding management of this challenging population are lacking. Therefore, we aimed to study the relationship between treatment strategies and outcomes following CD19-CAR-T failure.
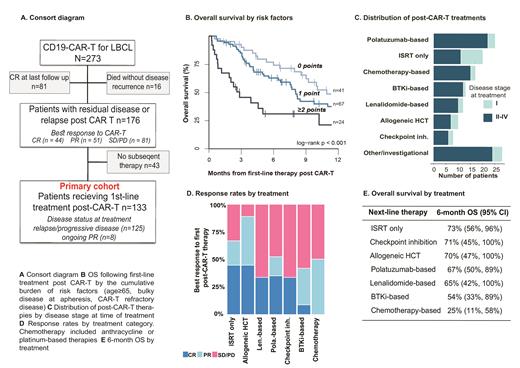
We included 273 adults, from two centers, treated with CD19-CAR-T (axicabtagene-ciloleucel [98, 36%], tisagenlecleucel [76, 28%], lisocabtagene-maraleucel [28, 10%], and an academic CD28-product [71, 26%]) for relapsed/refractory LBCL Cumulative incidence of relapse or progression was 40% (95% CI: 34%, 46%). Of 176 patients with residual or relapsed disease post-CAR-T (Fig. A), 133 received subsequent first-line anti-cancer therapy for active or residual disease (primary cohort) at a median of 79 days (interquartile range 49-124) after CAR-T infusion.
Within the primary cohort, 65% of patients had stage III-IV disease at time of subsequent therapy. Most lymphomas remained CD19-positive after CAR-T therapy (45 biopsies, 91% positive by flow cytometry [58% normal, 33% dim expression]). At time of first treatment post-CAR-T, nearly all patients had either relapsed disease or stable/progressive disease (SD/PD), with eight patients in ongoing partial response (PR).
With a median follow-up of 14.5 months (95% CI: 11.5-21.4), the median overall survival (OS) from time of first subsequent therapy was 8.6 months (IQR 6.9-12.0). We sought to identify determinants of survival among patients receiving initial post-CAR-T treatment. Variables measured pre- and post-CAR-T therapy and significantly associated (p < 0.1) with OS in univariable Cox regression were introduced into a multivariable model. Age ≥ 65y (HR 2.01 [95% CI: 1.23-3.29], p 0.005), bulky disease at apheresis (HR 2.05 [1.07-3.95], p 0.031), and disease refractory to CAR-T therapy (HR 1.89 [1.19-2.98], p 0.007) were associated with inferior OS in the multivariable analysis. Based on the cumulative burden of OS determinants, we propose a prognostic tool allowing risk stratification of patients receiving treatment post-CAR-T. Increasing number of these three risk factors was associated with greater mortality (HR 1.86 [1.32-2.62], p<0.001; Fig. B). Six-month OS ranged from 30% (95% CI: [16-57]) with ≥ 2 factors to 76% (64-91) with none.
Therapy strategies post-CAR-T varied. Polatuzumab (n=25), anthracycline or platinum ("chemotherapy"; n=17), BTK inhibitors (n=13) and lenalidomide (n=12) based treatment were most frequently administered for non-localized disease (stage ≥2). Involved site radiation therapy (ISRT; n=20) was primarily given for stage I disease (Fig. C). Overall response rate (ORR) in the entire cohort was 47% (25% CR; 22% PR). Fig. D shows response rates by treatment. Remarkably, novel agents, including polatuzumab and lenalidomide-based therapies, had ORR of 52% (CR 35%) and 33% (CR 33%), respectively. In contrast, traditional chemotherapy-based approaches did not result in CR, and only 50% achieved PR. Survival was poorest with chemotherapy (6 month OS: 25% [95% CI: [11-59]), while rates with lenalidomide and polatuzumab-based therapies were 65% (42-100) and 67% (50-89). Patients and disease characteristics across treatment groups were unbalanced. However, the three prognostic factors comprising the OS prognostic tool: age ≥ 65y, bulky disease at apheresis and disease refractoriness to CAR-T, were similar across lenalidomide, polatuzumab, checkpoint inhibitors, and chemotherapy-based treatment groups. Patients who underwent alloHCT were significantly younger but achieved high rates of response.
In conclusion, we present the most extensive and detailed experience of treatment outcomes post-CAR-T therapy. Our data suggest that novel agents may be preferable to traditional chemotherapies as the first post-CAR-T treatment. However, survival is still poor, and investigation of curative approaches is needed. We provide a tool to inform mortality risk in this difficult-to-treat population.
Scordo: i3 Health: Other: Speaker; Kite - A Gilead Company: Membership on an entity's Board of Directors or advisory committees; McKinsey & Company: Consultancy; Omeros Corporation: Consultancy; Angiocrine Bioscience: Consultancy, Research Funding. Batlevi: ADC Therapeutics: Consultancy; Juno/Celgene: Consultancy; Life Sciences: Consultancy; Regeneron: Current holder of individual stocks in a privately-held company; Karyopharm: Consultancy; Viatris: Current holder of individual stocks in a privately-held company; GLG Pharma: Consultancy; Xynomic: Research Funding; Seattle Genetics: Consultancy; Kite Pharma: Consultancy; TG Therapeutics: Consultancy; TouchIME: Honoraria; Memorial Sloan Kettering Cancer Center: Current Employment; Bayer: Research Funding; BMS: Current holder of individual stocks in a privately-held company; Medscape: Honoraria; Pfizer: Current holder of individual stocks in a privately-held company; Moderna: Current holder of individual stocks in a privately-held company; Dava Oncology: Honoraria; Roche/Genentech: Research Funding; Novartis: Research Funding; Epizyme: Research Funding; Janssen: Research Funding; Autolus: Research Funding. Dahi: Gilead sciences: Membership on an entity's Board of Directors or advisory committees; Kite pharma: Membership on an entity's Board of Directors or advisory committees. Giralt: PFIZER: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Actinnum: Membership on an entity's Board of Directors or advisory committees; SANOFI: Membership on an entity's Board of Directors or advisory committees; CELGENE: Membership on an entity's Board of Directors or advisory committees; AMGEN: Membership on an entity's Board of Directors or advisory committees; JANSENN: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; JAZZ: Membership on an entity's Board of Directors or advisory committees. Palomba: Pluto: Honoraria; Lygenesis: Honoraria; Magenta: Honoraria; Juno: Patents & Royalties; Wolters Kluwer: Patents & Royalties; WindMIL: Honoraria; Priothera: Honoraria; Nektar: Honoraria; Rheos: Honoraria; BeiGene: Consultancy; Seres: Honoraria, Other: Stock, Patents & Royalties, Research Funding; Kite: Consultancy; Ceramedix: Honoraria; Notch: Honoraria, Other: Stock; Novartis: Consultancy; PCYC: Consultancy. Salles: Ipsen: Consultancy; Regeneron: Consultancy, Honoraria; Genentech/Roche: Consultancy; Genmab: Consultancy; Takeda: Consultancy; Novartis: Consultancy; Incyte: Consultancy; Morphosys: Consultancy, Honoraria; Janssen: Consultancy; Epizyme: Consultancy, Honoraria; Allogene: Consultancy; Kite/Gilead: Consultancy; Loxo: Consultancy; Miltneiy: Consultancy; Debiopharm: Consultancy; Velosbio: Consultancy; Rapt: Consultancy; BMS/Celgene: Consultancy; Beigene: Consultancy; Abbvie: Consultancy, Honoraria; Bayer: Honoraria. Sauter: Genmab: Consultancy; Celgene: Consultancy, Research Funding; Gamida Cell: Consultancy; GSK: Consultancy; Bristol-Myers Squibb: Research Funding; Kite/Gilead: Consultancy; Precision Biosciences: Consultancy; Novartis: Consultancy; Spectrum Pharmaceuticals: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding. Shah: Amgen: Research Funding; Janssen Pharmaceutica: Research Funding. Avigdor: Gilead: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS: Research Funding; Janssen: Research Funding; Takeda: Consultancy, Honoraria. Perales: Bristol-Myers Squibb: Honoraria; Takeda: Honoraria; Equilium: Honoraria; Cidara: Honoraria; MorphoSys: Honoraria; Incyte: Honoraria, Other; Servier: Honoraria; Celgene: Honoraria; Medigene: Honoraria; Kite/Gilead: Honoraria, Other; Karyopharm: Honoraria; Nektar Therapeutics: Honoraria, Other; Merck: Honoraria; Novartis: Honoraria, Other; NexImmune: Honoraria; Miltenyi Biotec: Honoraria, Other; Omeros: Honoraria; Sellas Life Sciences: Honoraria. Shouval: Medexus: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal